Summary
Lyme disease is a complex illness which is characterized by a wide range of clinical symptoms that can significantly impact the quality of life for many patients. This study examines heterogeneity in symptom reporting in patients with well-defined post treatment Lyme disease (PTLD) in order to identify and characterize clinically relevant patient subgroups.
Why was this study done?
The objective of this study was to identify underlying subgroups with distinct symptom profiles within a heterogeneous group of patients with well-defined post treatment Lyme disease (PTLD) and to characterize and compare these subgroups across a range of demographic, clinical, and psychosocial factors.
How was this study done?
Study participants were recruited from a single-site Lyme disease referral clinic and evaluated by physical exam, clinical laboratory testing, and standardized symptom questionnaires. Two hundred and twelve participants met study criteria for PTLD, with medical record-confirmed prior treated Lyme disease as well as current symptoms and functional impact.
Participants were asked to self-administer a 36-item post-Lyme questionnaire of symptoms (PLQS) developed based on prior clinical and research experience among patients with PTLD. Participants indicated both presence and severity over the past 2 weeks for each symptom (0=absent, 1=mild, 2=moderate or 3=severe). Exploratory factor analysis (EFA) was performed to identify the relational structure of the symptoms included in the PLQS.
What were the major findings?
This study identified six symptom factors and three potentially clinically relevant subgroups among patients with well-characterized PTLD. The 6 factors are ‘Fatigue Cognitive’, ‘Ocular Disequilibrium’, ‘Infection-Type’, ‘Mood-Related’, ‘Musculoskeletal Pain’ and ‘Neurologic’. Symptoms were found to be clustered in seemingly physiologically relevant ways. For example, all three cognitive symptoms loaded onto the same Fatigue Cognitive factor, and joint pain, muscle pain and joint swelling loaded onto the Musculoskeletal Pain factor. The six factors also represented commonly recognized domains of the clinical presentation of patients with persistent symptoms in Lyme disease.
A latent profile analysis (LPA) was used to ultimately identify three patient subgroups corresponding to specific symptom profiles.
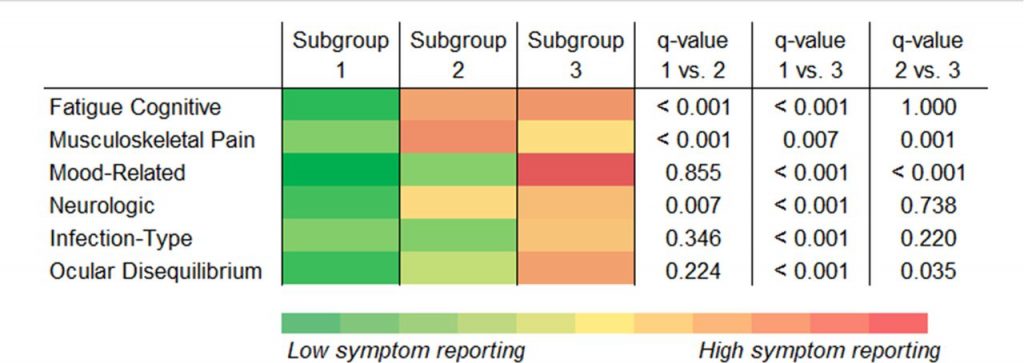
The 3 subgroups revealed additional patient heterogeneity. Subgroup 3 contained the highest symptom reporters, higher mood related factor scores, including depression, and higher prior neurologic pathology, including migraines. Subgroup 1 had the lowest symptom reporters, lower rates of disability, and higher reported self-efficacy. Subgroup 2 had the highest musculoskeletal pain.
Some slightly different correlations from a prior General Symptom Questionnaire-30 (GSQ-30) study in PTLD suggest that some symptoms, such as fatigue and insomnia, could arise from multiple factors. The 6 factors shed light on but do not necessarily equate to distinct biological mechanisms resulting in symptoms. Some symptoms may have a composite underlying mechanism, some may correlate with each another despite different mechanisms, and some distinct factors could represent different subtypes of a shared general mechanism. For example, difficulty breathing and heart palpitations can imply autonomic nervous system activation or central sensitization rather than specific cardiac or pulmonary pathology. As found in the prior GSQ-30 study, this study also showed PTLD extends beyond the effects of mood-related symptoms and that mood-related symptoms in PTLD may be more likely associated with fatigue or cognitive symptoms than with pain.
What is the impact of this work?
This analysis represents one of the first to identify and characterize potentially clinically relevant patient subgroups within the Lyme disease subset of post treatment Lyme disease (PTLD). This may serve as an important initial step towards analyzing the heterogeneity in symptom reporting that has long been observed among patients.
In order to methodically and credibly advance scientific understanding, a standardized, research definition for post-treatment Lyme disease (PTLD) is used to study a subset of patients with on-going symptoms linked to strong evidence of prior exposure to Borrelia burgdorferi. Unfortunately, lack of physician education has led to misuse of the term PTLD as meaning “post infection” despite the highly specific research definition of PTLD clearly meaning “post initial treatment”, without meaning any implied infectious or post infectious cause. Prior rigorous evidence-based peer reviewed research has showed the causes of PTLD can be multifactorial and vary person to person and this heterogeneity is often compounded by the significant impact of persistent symptoms on patient quality of life and functioning.
The presence of chronic or persistent symptoms following acute infection has been documented in subsets of patients for a number of viral and bacterial pathogens. Similar to fibromyalgia and ME/CFS, and the newly described long haul COVID-19, PTLD is a complex, multifactorial illness with immunologic, microbiologic, genetic and/or psychosocial factors contributing to disease development, severity, and persistent symptoms such as fatigue, musculoskeletal pain, and cognitive dysfunction.
This study validates the symptom heterogeneity that has long been observed among patients with persistent symptoms in Lyme disease. Insights from clinically and/or biologically coherent Lyme disease subgroups may help improve the understanding of complex causes and disease mechanisms and lead to novel treatment approaches to address the varied and/or multiple factors which contribute to illness perpetuation.
This research was supported by:
- The Steven and Alexandra Cohen Foundation
- RDRCC / P30
Publication Information
Rebman AW, Yang T, Aucott JN. Symptom heterogeneity and patient subgroup classification among US patients with post-treatment Lyme disease: an observational study BMJ Open 2021;11:e040399. doi: 10.1136/bmjopen-2020-040399
This research was funded by:
The Steven and Alexandra Cohen Foundation and a RDRCC/P30 grant AR070254


